FAIR USE NOTICE: This
document contains copyrighted material whose use has not been specifically
authorized by the
copyright owner. This material is available as part of
a mission to promote critical thinking about chemistry and chemical issues
in a
current social and topical context. The use is patterned
after CHANCE, a
well established program in Statistics.We believe that this
constitutes a 'fair use' of the copyrighted material
as provided for in section 107 of the US Copyright Law. If you wish to
use this
copyrighted material for purposes of your own that go
beyond 'fair use', you must obtain permission from the copyright owner.
Copyright 2001, The American Chemical Society
SCIENCE
CONCENTRATES
March
12,2001
Volume 79,
Number11
CENEAR 79
11 pp.53
ISSN 0010-2347
Terpene
isonitriles bind heme
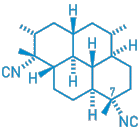 The
antimalarial activity of the compound shown, isolated from a marine sponge,
stems from its ability to coordinate with pure heme, a recent study shows
[J.
Med. Chem., 44, 873 (2001)]. Pure heme is formed when the
malaria parasite feeds on hemoglobin. The product is toxic to the parasite,
which disposes of it in various ways. The well-known quinoline antimalarials
prevent these detoxification processes. Now, researcher Anthony D. Wright
and others at the University
of Bonn, in Germany; La
Trobe University in Bundoora, Australia; and Heinrich-Heine University
in Dusseldorf, Germany, have found that compounds of the type shown--terpene
isonitriles--achieve the same effect to different degrees by forming a
complex with pure heme. The complex prevents destruction of pure heme,
allowing it to build up and damage proteins and lipids in the parasite.
Through modeling studies and structure-activity analysis of a series of
terpene isonitriles, the researchers establish that, in this compound class,
a lipophilic core comprising at least a tricyclic frame and carrying an
axial isonitrile group at C-7 is associated with the ability to bind pure
heme.
The
antimalarial activity of the compound shown, isolated from a marine sponge,
stems from its ability to coordinate with pure heme, a recent study shows
[J.
Med. Chem., 44, 873 (2001)]. Pure heme is formed when the
malaria parasite feeds on hemoglobin. The product is toxic to the parasite,
which disposes of it in various ways. The well-known quinoline antimalarials
prevent these detoxification processes. Now, researcher Anthony D. Wright
and others at the University
of Bonn, in Germany; La
Trobe University in Bundoora, Australia; and Heinrich-Heine University
in Dusseldorf, Germany, have found that compounds of the type shown--terpene
isonitriles--achieve the same effect to different degrees by forming a
complex with pure heme. The complex prevents destruction of pure heme,
allowing it to build up and damage proteins and lipids in the parasite.
Through modeling studies and structure-activity analysis of a series of
terpene isonitriles, the researchers establish that, in this compound class,
a lipophilic core comprising at least a tricyclic frame and carrying an
axial isonitrile group at C-7 is associated with the ability to bind pure
heme.
Top
Even
ordinary proteins can change shape
Under the right conditions of temperature, salt concentration,
and pH, even a globular protein like myoglobin will lose its structure
and convert to threadlike fibrils "that closely resemble the amyloid and
prion aggregates seen in pathological conditions such as Alzheimer's and
Creutzfeldt-Jakob disease," according to Oxford
University chemists Marcus Fändrich, Matthew A. Fletcher, and
Christopher
M. Dobson [Nature, 410, 165 (2001)]. Their experiments
with the protein portion of myoglobin (without its heme group) suggest
that any polypeptide is susceptible to radical rearrangement under the
right conditions. Thus the amino acid sequences of the polypeptides associated
with amyloid or prion diseases may not be particularly important. They
propose that native proteins have protective mechanisms, such as cooperativity
in folding and molecular chaperones, that ordinarily suppress amyloid formation.
Conditions that compromise these protective measures--including aging,
mutations, and, in the case of prion diseases, ingestion of infectious
material--could cause proteins to convert to a fibrillar conformation.
 The
antimalarial activity of the compound shown, isolated from a marine sponge,
stems from its ability to coordinate with pure heme, a recent study shows
[J.
Med. Chem., 44, 873 (2001)]. Pure heme is formed when the
malaria parasite feeds on hemoglobin. The product is toxic to the parasite,
which disposes of it in various ways. The well-known quinoline antimalarials
prevent these detoxification processes. Now, researcher Anthony D. Wright
and others at the University
of Bonn, in Germany; La
Trobe University in Bundoora, Australia; and Heinrich-Heine University
in Dusseldorf, Germany, have found that compounds of the type shown--terpene
isonitriles--achieve the same effect to different degrees by forming a
complex with pure heme. The complex prevents destruction of pure heme,
allowing it to build up and damage proteins and lipids in the parasite.
Through modeling studies and structure-activity analysis of a series of
terpene isonitriles, the researchers establish that, in this compound class,
a lipophilic core comprising at least a tricyclic frame and carrying an
axial isonitrile group at C-7 is associated with the ability to bind pure
heme.
The
antimalarial activity of the compound shown, isolated from a marine sponge,
stems from its ability to coordinate with pure heme, a recent study shows
[J.
Med. Chem., 44, 873 (2001)]. Pure heme is formed when the
malaria parasite feeds on hemoglobin. The product is toxic to the parasite,
which disposes of it in various ways. The well-known quinoline antimalarials
prevent these detoxification processes. Now, researcher Anthony D. Wright
and others at the University
of Bonn, in Germany; La
Trobe University in Bundoora, Australia; and Heinrich-Heine University
in Dusseldorf, Germany, have found that compounds of the type shown--terpene
isonitriles--achieve the same effect to different degrees by forming a
complex with pure heme. The complex prevents destruction of pure heme,
allowing it to build up and damage proteins and lipids in the parasite.
Through modeling studies and structure-activity analysis of a series of
terpene isonitriles, the researchers establish that, in this compound class,
a lipophilic core comprising at least a tricyclic frame and carrying an
axial isonitrile group at C-7 is associated with the ability to bind pure
heme.