 Chem 121: Assignments
Chem 121: Assignments
Reading / Homework / Slides,Workshops & Worksheets / Labs & Reports
Chem 121: Assignments
Reading / Homework / Slides,Workshops & Worksheets / Labs & Reports
Course Outline & Learning Objectives:
http://chemconnections.org/general/chem121/CHEM-121.cor.pdf
http://chemconnections.org/general/chem121/CHEM-121.slos.pdfRead and understand the material & concepts in all Zumdahl sections highlighted in bright red.
1. Acid base review
14.1 The Nature of Acids and Bases
14.2 Acid Strength
14.3 The pH Scale
14.4 Calculating the pH of Strong Acid Solutions
14.5 Calculating the pH of Weak Acid Solutions
14.6 Bases
14.7 Polyprotic Acids
14.8 Acid-Base Properties of Salts
14.9 The Effect of Structure on Acid-Base Properties
14.10 Acid-Base Properties of Oxides
14.11 The Lewis Acid-Base Model
14.12 Strategy for Solving Acid-Base Problems: A Summary
SolutionsAcids, Bases and Equilibrium Concept
* Ionization Constants
* Bronsted Concept of Acids and Bases
* Monoprotic versus Polyprotic Acids and Bases
* Conjugate Acid-Base Pairs
Water and the pH Scale
* Water Autoionization and the Water Ionization Constant, Kw
* pH Scale
* Determining and Calculating pH
Equilibrium Constants for Acids and Bases
* Ka and Kb Constants
* Aqueous Solutions of Salts
* A Logarithmic Scale of Relative Acid Strength, pKa
* Relating the Ionization Constants for an Acid and Its Conjugate Base
* Predicting the Direction of Acid-Base Reactions
* Neutralization
Calculations with Equilibrium Constants
* Calculating a Ka value from a Measured pH
* Calculating Equilibrium Concentrations and pH from Ka
* What is the pH of an Aqueous Solution of a Weak Acid or Base?
* What is the pH of the Solution After an Acid-Base Reaction?
* Polyprotic Acids and Bases
* Calculating the pH of the Solution of a Polyprotic Base/Acid
* Lewis Concept of Acids and Bases
Molecular Structure, Bonding and Acid-Base Behavior
* How to predict the relative strength of Acids and Bases
Acid-Base Titrations
* Titration of a Strong Acid with a Strong Base
* Titration of a Weak Acid with a Strong Base
* pH Indicators
2. Buffers and titration curves
Acid-Base Equilibria
15.1 Solutions of Acids or Bases Containing a Common Ion
15.2 Buffered Solutions
15.3 Buffer Capacity
15.4 Titrations and pH Curves
15.5 Acid-Base Indicators
15.6 Solubility Equilibria and the Solubility Product
Solutions
# Ionic equilibria
3. Solubility product
4. Selective precipitation
5. Dissolving precipitates
6. Formation constants for complex ions
15.6 Solubility Equilibria and the Solubility Product
15.7 Precipitation and Qualitative Analysis Complex Ion Equilibria
15.8 Equilibria Involving Complex Ions
Solutions
1. Three laws of thermodynamics
2. Gibbs free energy
3. Calculation of equilibrium constants
16.1 Spontaneous Processes and Entropy
16.2 Entropy and the Second Law of Thermodynamics
16.3 The Effect of Temperature on Spontaneity
16.4 Free Energy
16.5 Entropy Changes in Chemical Reactions
16.6 Free Energy and Chemical Reactions
16.7 The Dependence of Free Energy on Pressure
16.8 Free Energy and Equilibrium
16.9 Free Energy and Work
Solutions
1. Electrochemical cells
2. Electrode potential
3. Nernst equation
4. Electrolysis and Faraday?s laws
17.1 Galvanic Cells
17.2 Standard Reduction Potential
17.3 Cell Potential, Electrical Work, and Free Energy
17.4 Dependence of Cell Potential on Concentration
17.5 Batteries
17.6 Corrosion
17.7 Electrolysis
17.8 Commercial Electrolytic Processes
Solutions
9.1 Hybridization and the Localized Electron Model
9.2 The Molecular Orbital Model
9.3 Bonding in Homonuclear Diatomic Molecules
9.4 Bonding in Heteronuclear Diatomic Molecules
9.5 Combining the Localized Electron and Molecular Orbital Models
Solutions
1. Formation and structure of complexes
2. Isomerism
3. Crystal field theory
21. Transition Metals and Coordination Chemistry
21.1 The Transition Metals: A Survey
21.2 The First-Row Transition Metals
21.3 Coordination Compounds
21.4 Isomerism
21.5 Bonding in Complex Ions: The Localized Electron Model
21.6 The Crystal Field Model
21.7 The Biologic Importance of Coordination Complexes
21.8 Metallurgy and Iron and Steel Production
Solutions
1. Structure and isomerism
2. Classification of the basic functional groups
# Spectroscopy - absorption spectra, visible, UV, IR, NMR
22.1 Alkanes: Saturated Hydrocarbons
22.2 Alkenes and Alkynes
22.3 Aromatic Hydrocarbons
22.4 Hydrocarbon Derivatives
22.5 Polymers
22.6 Natural Polymers
Solutions
1. Factors affecting reaction rate
2. Collision and transition state theories
3. Arrhenius equation
4. Rate laws
5. Reaction mechanism
12.1 Reaction Rates
12.2 Rate Laws: An Introduction
12.3 Determining the Form of the Rate Law
12.4 The Integrated Rate Law
12.5 Rate Laws: A Summary
12.6 Reaction Mechanisms
12.7 A Model for Chemical Kinetics
12.8 Catalysis
Solutions
1. Nuclear binding forces, nuclear instability
2. Radioactivity, nuclear equations including fission and fusion
3. Mass energy relationships
18.1 Nuclear Stability and Radioactive Decay
18.2 The Kinetics of Radioactive Decay
18.3 Nuclear Transformations
18.4 Detection and Uses of Radioactivity
18.5 Thermodynamic Stability of the Nucleus
18.6 Nuclear Fission and Nuclear Fusion
18.7 Effects of Radiation
SolutionsChem 121: ChemWiki
* The discovery of radioactivity and its first applications
* Types of nuclear reactions
* Radioactive decay rates
* Nuclear stability and magic numbers
* Artificially induced radioactivity
* Energetics of nuclear reactions
* Effect of radiation on matter
* Fission and fusion
Case Studies
* Radiocarbon dating
* Positron emission tomography
* Nuclear Weapons
* Nuclear Reactors
* Nuclear Waste
* Chernobyl
* Nuclear Stability and Magic Numbers
* Fission and Fusion
* Case Studies
* Radioactivity
* Nuclear Stability and Magic Numbers
* Fission and Fusion
* Case Studies
* Radioactivity
1. Properties and qualitative analysis of selected ions
2. Application of principles of ionic equilibrium, redox, and complex ion formation to the separation and detection of ions.
# Quantitative analysis
1. Quantitative spectrocopic instrumentation: eg. AA, UV/Vis
2. Calibration curves, statistical analysis of data19. The Representative Elements: Groups 1A Through 4A
19.1 A Survey of the Representative Elements
19.2 The Group 1A Elements
19.3 Hydrogen
19.4 The Group 2A Elements
19.5 The Group 3A Elements
19.6 The Group 4A Elements
20. The Representative Elements: Groups 5A Through 8A
20.1 The Group 5A Elements
20.2 The Chemistry of Nitrogen
20.3 The Chemistry of Phosphorus
20.4 The Group 6A Elements
20.5 The Chemistry of Oxygen
20.6 The Chemistry of Sulfur
20.7 The Group 7A Elements
20.8 The Group 8A ElementsALEKS homework problems are required. These are to be completed before each exam that covers the particular chapter. There will be no possibility of completing the assignment or gettting any credit after that deadline.
Class Slides, Workshops & Worksheets:
- Thermochemistry:
- Calculations Guide pdf
- Electrochemistry:
Kinetics pdf
Molecular Orbitals .pdf
To be completed before tomorrow's class. 5 pt. quiz grade; a zero will be entered if the deadline is missed. 5 pts will be deducted from all subsequent quizzes for each day missed until an e-mail is sent.
You must use an e-mail account that is
your OWN personal account; one that Dr R. can absolutely rely
on to contact you. Otherwise you will not receive important information.
Address your e-mail to: rrusay@chemconnections.org
Title the e-mail: Chem 121 f-2011
In the body of the e-mail message provide the following information.
- Educational Opportunities 2. Pensions / Social Security 3. OTHER: Caring for elderly family
A. Agricultural Production
B. Art, Literature and Music
C. Climate Change
D. Communications / Networking
E. Crime
F. Depletion of Natural Resources
G. Economic Growth
H. Educational Opportunities
I. Energy Production
J. Environmental Pollution
K. Health Care
L. Insurgencies
M. Jobs
N. Manned Space Exploration
O. Pensions / Social Security
P. Population Growth
Q. Religious Intolerance
R. Rising Sea Levels
S. Scientific Literacy
T. Social Injustice
U. Technical Innovation
V. Terrorism
W. OTHER: (Please Name)
(Optional) Learning styles survey
http://chemconnections.org/general/chem121/learning-11.html
If you do take the Survey and/or the Keirsey Temperament Sorter, include the results in your e-mail, eg. Survey: V=15 A=30 T=20; Keirsey = ISTJ.
Write clearly & legibily in your laboratory notebook. These pages
must be included in your final report.
Prelab & Final Lab Reports:
Pre-Lab reports must be prepared prior to each scheduled lab. The pre-lab
report must be in a format that includes: Title, Purpose, Procedure (can
be referenced
or outlined), Chemical
Reactions (where appropriate), and when required, neatly organized,
labeled, blank Data Tables with the units of measurement,
eg. grams, mL, etc. plus any assigned pre-lab
questions.
Before you begin any lab, you must have Dr. R. initial the lab
notebook
page with the pre-lab report.
The final, complete Lab report
must include all pertinent information including unknown numbers where
unknowns
are part of the experiment and a Results/Conclusions section plus any
post lab questions.
The raw lab notebook pages may be submitted as your final report or you may re-write the report using a wordprocessor and attach the lab notebook pages.
|
|
|
Data:
Report the equivalence point volume and pH: (A table would be best.)
1) From the titration curve.
2) From either the first or second derivative of the titration curve. (Identify which you are using.)Calculations: (Show your calculations)
Using the equivalence point data from the titration curve.
1) Calculate the exact molarity of the ~0.1M unknown acid solution before titration began.
2) Calculate Ka: SEE: Calculation Example
Using the data at the 1/2 way titration point volume (or a point near to it).
3) Calculate Ka
Results & Post Lab Questions:
1) Identify your unknown acid from the choices next to the example of an experimental titration curve above. Write its chemical formula.
2) (a) Find and clearly report the Ka for your unknown acid from a reliable reference source. (b) Calculate the % error for your respective values from calculation 2) and 3) above. (Use the reliable reference value as the "correct" value.)
3) List possible sources of error in the experiment. Select one that would likely be the most significant. Also, would using the data from the first or second derivative improve the accuracy. Briefly explain.
4) Label four regions on your printed titration curve. In each region clearly describe the relative concentrations of the unknown acid (un-ionized), hydroxide ion, hydronium ion, and the conjugate base. (Suggestion: A table would likely provide the clearest way of presenting your description of what is happening as to the relative concentrations in each region.)
5) Calculate the percent difference between the volume at the equivalence point and the end point volume. If the end point volume were used instead of the equivalence point volume, would there be a significant effect on Ka error (higher or lower than actual?). Briefly explain your answer.
6) Group Workshop: Titration Curves (Turn in one per group.)
Buffers (Read pp.19-20 of the lab manual) Find your assigned buffer pH. See: buffer list
First consult Table A in your lab manual pp.111 & 112. Select a buffer system using one of the following solids that will provide a pKa close to the assigned pH:
In your lab notebook:
Prepare a pre-lab report that includes: 1) Title, 2) Purpose, 3) Chemical Reactios for the buffer system that you have chosen for the assigned pH.
a ) The salt in equilibrium with either its conjugate acid or its conjugate base and b) the reaction of HCl or NaOH with the salt
(Have Dr. R. initial and grade.) Grading Rubrik4) Calculations: (Clearly show the calculations.)
Calculate: 1) the mass of solid to weigh out to prepare 100.mL of a buffer solution (Where the least concentrated component is 0.1M.) and 2) the volume of standardized 0.1M NaOH or 0.1M HCl solution necessary to react with the solid.5) Procedure: A brief outline of what you plan to do using the results of your calculations.
(Have Dr. R. initial and grade before preparing the buffer solution.)6) Data Tables
Prepare the buffer. Test the pH, and record under Results section in lab notebook.
Have Dr. R. evaluate the buffer's performance and its capacity.Post Lab
Group Workshop
Buffers
Bonus:
Blood & Oceans Reading
Blood & Oceans WKS
- Blood pH/ Buffers Presentation
- Ocean pH/ Buffers Presentation
Ionic Reactions (Read pp.25-29 of the lab manual) (Foundation for Qualitative Analysis)
Safety: Handling thioacetamide / H2S
A major concern for each of us this semester is using thioacetamide safely, and handling metal sulfides conscientiously and carefully, since they produce hydrogen sulfide. Exposure to hydrogen sulfide, which is generated from thioacetamide and from the acid treatment of metal sulfide precipitates, is as any other chemical, toxic at certain concentration levels. The toxicity levels and effects of hydrogen sulfide are:
- 0.0047 ppm is the smell threshold, the concentration at which 50% of humans can detect the characteristic rotten egg odor of hydrogen sulfide
- 10-20 ppm is the borderline concentration for eye irritation.
- At 150-250 ppm the sense of smell disappears, and with it the awareness of danger,
- 800 ppm is listed as the lethal concentration for 50% of humans for 5 minutes exposure (LC50).
Our aim is to minimize exposure and keep hydrogen sulfide well below 10 ppm in PS 213. The individual student hoods in PS 213 are ineffective to do this when too many are in use, and there is not enough large hood space to meet our class needs. If the individual fume hoods on the lab benches are limited to only 7 hoods in-use and spread evenly throughout the lab with the other vents closed, it will keep exposures to a minimum providing that each of us handle the chemicals properly.
Use thioacetamide only in a hood. Produce, transfer and treat all metal sulfides in a hood, including centrifuging and decanting. If you transport metal sulfides in the open lab or have them sitting in your test tube rack, be sure to cover the test tubes with parafilm so that it forms a good seal.
Science 19 August 2011:
Vol. 333 no. 6045 pp. 922-923
DOI: 10.1126/science.333.6045.922-b
Write clearly & legibily in your laboratory notebook. These pages may be submitted as your final report or you may re-write the report using a wordprocessor and attach your lab notebook pages.
Prelab & Final Lab Report:
The report must be in a format that includes: Title, Purpose, Procedure (can be referenced or outlined), Data (Tables as follow) and NIEs (Net Ionic Equations) following the lab manual's instructions with the changes/ exceptions as noted below. Use pp. 30-40 from Lab Manual for NIEs for those reactions that are noted.
[Omit Results/Conclusions Section]
|
LAB REPORT: The Net Ionic Equations (NIEs) that must be included in the report are listed below. Turn in stapled to your lab notebook pages.
[Omit Results/Conclusions]
NIEs: Use pp.30-40 from DVC Lab Manual for the following NIEs.
Part 1: Only observed reactions
Part 2: Only observed reactions: precipitation and dissolution where observed
Part 3: Only observed reactions: precipitation and dissolution where observed
Part 4: All reactions: OMIT Hg2+(aq)
Part 5: OMIT Hg2+(aq)
Presentation
Presentation .pdfRefer to the procedures in Slowinski, Qualitative Analysis, Group I: pp. 37-40 & Group II pp.51-60, (NOTE the changes in the Group II procedure (Changes Group II .pdf)
Also see Flow Charts: Group I .pdf; Group II .pdf
Prelab I/II: Provide a Flow Chart with reagents for the following ions, Pb2+ Ag+ Cu2+ Bi3+ Sb3+The following materials were adapted from and with links to the pages of Professor Jim Bert's Chem 115 course, Arizona State University
General reactions and properties of some qual cations
Confirmatory tests
TechniquesFinal REPORT: Lab Notebook pages only.
1) Notebook pages must have observations clearly stated and accompany the Flow Chart(s) 2) NIEs equations must be included for ALL reactions of the ions present in the unknown sample in the Results section.
3) All of the possible ions should be clearly written in the Results section with those present circled.Group II/III: Prelab provide a Flow Chart with reagents / references for the following ions, Pb2+ Cu2+ Bi3+ Sb3+ Cr3+ Mn2+ Fe3+ Ni2+ Zn2+ Co2+ Al3+
Presentation / i-clicker questions (.pdf)
Final REPORT: Lab Notebook pages only.
1) Notebook pages must have observations clearly stated and accompany the Flow Chart(s) 2) NIEs equations must be included for ALL reactions of the ions present in the unknown sample in the Results section.
3) Turn-in FormPost lab Reading & Questions: Iron Mountain, California (Printable Page)
http://en.wikipedia.org/wiki/Iron_Mountain_Mine
Iron Mountain Mine, also known as the Richmond Mine at Iron Mountain, is a mine near Redding in Northern California. Geologically classified as a "massive sulfide ore deposit", the site was mined for iron, silver, gold, copper, zinc, and pyrite intermittently from the 1860s until 1963. The mine is the source of extremely acidic mine drainage which also contains large amounts of zinc, copper and cadmium. One of America's most toxic waste sites, it has been listed as a federal Superfund site since 1983.
The drainage water from the Iron Mountain Mine is the most acidic water on Earth; some samples collected in 1990 and 1991 have been measured to have a pH value of -3.6, which is the lowest pH observed globally in an natural environment.
When pyrite is exposed to moisture and oxygen, sulfuric acid forms. This sulfuric acid runs through the mountain and leaches out copper, cadmium, zinc, and other heavy metals. This acid flows out of the seeps and portals of the mine. Much of the acidic mine drainage ultimately is channeled into the Spring Creek Reservoir by creeks surrounding the mine. The low pH level and the heavy metal contamination from the mine have caused the virtual elimination of aquatic life in sections of Slickrock Creek, Boulder Creek, and Spring Creek.
The mine was designated a Superfund site in 1983 and a water treatment plant was built in 1994. In 2000 the government reached a settlement with the responsible parties for the long-term funding of the cleanup efforts, which will require ~ 1 billion dollars.Acid mine drainage biogeochemistry at Iron Mountain, California
Gregory K Druschel, Brett J Baker, Thomas M Gihring, and Jillian F Banfield
Geochem Trans. 2004; 5(2): 13
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1475782/Post Lab Questions:
Large natural deposits of iron pyrite, "fool's gold", which has the unusual molecular formula FeS2 is Fe2+S22-, where the sulfur is a diatomic anion with a covalent S–S bond. Therefore, pyrite is correctly named as iron (II) persulfide and not iron (II) sulfide as seen in some references.
Sulfuric acid is formed naturally by the oxidation of iron (II) persulfide. The resulting water is highly acidic and is called acid mine drainage (AMD) or acid rock drainage (ARD). This acidic water is capable of dissolving other metal minerals present in sulfide ores, which produces brightly colored (usually blood red), streams. They are acidic with high concentrations of metals that are toxic to fish and aquatic organisms. The oxidation of pyrite, iron (II) persulfide, with molecular oxygen produces iron(II), Fe2+, and sulfuric acid.
1) Write a balanced Net Ionic Equation for the redox reaction:
Fe2+ is further oxidized with oxygen to Fe3+ under acidic conditions
2) Write a balanced Net Ionic Equation for the redox reaction:
The Fe3+ produced can be precipitated in water as the hydroxide.
3) Write a balanced Net Ionic Equation for the precipitation reaction:
4) Write the equlibrium reaction and the solubility product expression for the solubility of iron(III) hydroxide:
Iron(III) ion oxidizes pyrite in water to produce sulfuric acid and iron (II) ion.
5) Write a balanced Net Ionic Equation for the redox reaction:
a) How many electrons are transferred in the process?6) The rate of weathering /dissolution of iron pyrite(Reaction #1), which is an exothermic process is relatively slow, but the dissolution rate increases dramatically with iron pyrite reacting with iron(III) ion, (Reaction #5), which is also exothermic. Draw an energy diagram illustrating the reactions on the same diagram and describe the respective differences in their Energy of Activation.
7) Using chemical equations, solubility principles, Ksp, and pH, briefly explain how pH control in the Qual Separation Scheme for separating Group 2 from Group 3 metal ions as their sulfides relate to the leaching of iron and copper into the streams that feed the Sacramento River.
Atomic Absorbtion (AA) analysis of a statistically significant number of samples of streams near Iron Mountain's water outfall resulted in an average concentration that suggests 1.5 x 104 moles of iron pyrite dissolve per day.
8) Using the AA results calculate how many tons (1 ton = 1,000 kg) of iron pyrite dissolve per year and how many tons of iron(II) ion is added to the streams and river per year. Show your calculations.
The chemistry of limestone cave formation is similar in some ways to the remediation steps taken to treat the toxic mine waste.
9) Describe the similarities using chemical equations.
Virtual ChemLab Qualitative Analysis Student Guide (pdf format)
The following materials were adapted from and with links to the pages of Professor Jim Bert's Chem 115 course, Arizona State University
General reactions and properties of some qual cations
Confirmatory tests
Techniques
|
|
|
|
|
|
|
Flow Charts
|
Handout
|
Handout
|
Handout
|
Handout
|
|
Procedures
|
HCl |
Refer to references.
|
Refer to references.
|
Group I/II:
1) Complete Introduction to Qualitative Analysis : Virtual Chem Lab (Prelab)
2) After turning in individual prelab forms obtain an individual Group I/II lab unknown from Dr. R. (Unknowns have 3 or 4 ions)VIRTUAL CHEM LAB I/II
Login using the Virtual Chem Lab icon on the computers in PS 110 or the e-Macs in the instrument room, PS-211, or certain Chem i-books.
Open Virtual Chem Lab
Click on General Chemistry Laboratory
Click on Add New User
User Name: (Case Sensitive!) your last name plus first initial of first name, eg. for Barry Bonds: BondsB
Password: DVC id without leading zeroes
Click on Inorganic Bench: Lab Book : Enable Web Connection
Enter: Enable Web Connection
Enter: Your Name and Password
Enter URL: http://192.235.0.133:8080/vchemlab/servlet/ChemServ
Enable Automatic Updates
Enter: Save
Go Back and re-enter Virtual Chem Lab
NOTE: If you reuse any computer that you have previously logged onto as above, you only need to enter your password and then OK to login.After re-entering go to Inorganic Stockroom to pick up your unknown solution.
Record results in lab book, be sure to save on server.
Submit when certain of ions on or before the due date. Cannot be changed once submitted.Group II/III:
Do #1) and #2) in parallel:
1) Complete Virtual Chem Lab (Unknowns have 4 ions).
2) Obtain individual unknown from Dr. R. (Unknowns have 4 ions: There is at least one ion from each group. Cadmium is not present.)VIRTUAL CHEM LAB
Individual unknowns have 4 cations from GroupsII/III out of a possible 12:
Al3+, Bi3+, Co3+, Cr3+, Cu2+, Fe2+, Hg2+, Mn2+, Ni2+, Sb3+, Sn4+, Zn2+Reminder:
Add New User on opening page or if necessary from Inorganic Bench: Lab Book /Enable Web Connection
User Name: (Lower Case) last name plus first initial of first name, eg. for Barry Bonds: bondsb
Password: DVC id without leading zeroes
URL: http://192.235.0.133:8080/vchemlab/servlet/ChemServ
Enter: Save
Go Back and re-enter Virtual Chem LabGroup I/II/III/IV: (General Unknown)
1) Virtual Chem Lab Unknown (Unknowns have 6 ions: There may not be any ion from one of Groups I-IV. There is one ion from Group V including ammonium as a possibility. Cadmium is not not present.)
2) Individual General Lab Unknown (Unknowns have 5 ions: There may not be any ion from one of the groups. There may be more than one ion from a Group. Cadmium is not present. There are no ions from Group V. There are one or more ions from Group IV. )REPORTS:
1) Notebook pages must have observations clearly stated. Flow Chart (handout) for unknown Groups must be included.
2) NIEs equations must be included for ALL reactions of the ions in the unknown sample.
3) An "Unknown" Flow Chart for the ions present in your sample must be developed and included in your lab notebook under RESULTS. It must clearly show how to separate & distinguish between the actual ions present in the unknown sample once those ions are known to you: (Omit the unnecessary parts of the flow chart in the handout.)
Ksp / Thermodynamics / Spreadsheet Applications
"Temperature Dependence of the Solubility of Ba(IO3)2"Titrations:
Refer to Lab Manual. A minimum of 3 replications (must be within a precision of +/- 1%) of a solution of Ba(IO3)2 at the one temperature, which was assigned to you.

Processing Data:
1) Solve for the Ksp at your assigned temperature.Refer to Dr Ruehl's flash videos on the use of Excel:
A) Copying formulas
B) Plotting Graphs
2) Using your assigned group members' data plot ln Ksp vs. 1/T(K)
3) Using the combined class data, plot ln Ksp vs. 1/T(K) on the same graph
Final Report, in standard format, is to include the following information plus other pertinent, routine report data (balanced equations, etc):
1) Provide a print out with your name and all of your group members' names that has the spreadsheet data table used for generating the figure (graph) with a straight lines drawn including correct labels and the R2 value. Attach to Understanding Solubility page.
2) Include calculations with respective examples showing how to determine:
- Precision of your titrations (+/- __ mL); Must be = or < 1% of the average volume.
- The values for Ksp for your temperature, ΔH, and ΔS from your group's data.
- % Error: (1) % differences between group experimental values and accepted class values.
4) Results:
- Error analysis (Discuss the factors that relate to the percent difference between experimental and accepted class values for ΔH and ΔS and Ksp.
5) Post Lab / Group Workshop: Thermochemistry-Equilibrium
6) Understanding Solubility: Preparing Graphs & Spreadsheets with Excel (A) Instructions .pdf & (B) Worksheet Questions .pdf ; (C) Data.html (D) Excel Data download .xls
Class Data 2011 Group Data and Accepted Data for Error Analysis
Electrochemistry (Table of Standard Reduction Potentials)
Pre-Lab: In your Lab notebook outline the procedure for each of the five parts of the experiment. Select 2 lab partners that have completed the procedure's outline. Get Dr. R's initials and an individual unknown for each partner. After getting the unknown, with your partners complete the procedure for Part 5: Corrosion before leaving the lab today. The rest of the lab report will be done on pp. 54-58 pf the lab manual.
Day 2: Either remain with your partners or reorganize into groups of two-three and complete Parts 1, 2 and 4. Part 3 is to be done individually. An individual report will be submitted by everyone using pages 54-58 from the Lab manual. List your partner(s) on the report after your name. Attach your pre-lab notebook pages. Answer questions a) and b) below with your partner(s) and attach to the final report.
1) Part 1: Reducing Potentials (Tables #1 and #2)
2) Part 2: Effect of Concentration
3) Part 3: Ksp of Unknown Silver Salt
4) Part 4: Electrolysis
5) Part 5: Corrosion
 |
 |
 |
| a) DVC has an electric charging station. It appears that no one
uses it. What automobiles are currently on the market that could
use it and
what
are their range is prices?
b) Sketch an example of a fuel cell that includes its chemical reactions. Are the reaction products from this fuel cell different than the products from combustion from the same reactants? What makes the energy derived from fuel cells different from that derived from combustion? |
|
Electrochemical Restoration of Sunken Treasure
Calibrated Peer Review (CPR) Individual Writing Assignment: http://cpr.molsci.ucla.edu/
BEWARE of PlagiarismIn this assignment, you will:
• Explore a web site that describes the wreck of the ship "Atocha," and describes some of the silver coins and other objects found in the shipwreck.
• Read a passage that describes the chemistry that caused a layer of silver sulfide to form on the silver objects, and do further reading in your textbook on redox reactions, oxidation states, electrochemical principles, and electron transfer.
• Formulate a plan to electrochemically remove the layer of silver sulfide from the silver object.
• Write an essay that describes how you would use electrochemistry to "clean" the silver treasures from the "Atocha."1) Take the Tour.
2) For first time login: Select Diablo Valley College from Institutions, login using your DVC id for initial login, record your new CPR id and change your password.
3) Begin assignment following the scheduled due dates in the course calendar.
 |
 |
|
 |
 |
Beer's Law: Concentration of a Ni2+ unknown, Transition metal complexes.
Final Report is to be in standard format including data, a Beer's Law plot that is submitted with your unknown data in a print version.
Atomic Absorption Spectroscopy (Quantitative Analysis)
Background .pdf
Analysis of a vitamin supplement.
Procedure .pdf
Prelab .pdf
Report Form .pdf
ppm & ppb can be mass solute/ mass solution
or
can be mass solute/ volume solution:ppm = [mass solute/ volume solution] x 106
ppm = mg / L = 0.001 g / 1000 mlppb = [mass solute/ volume solution] x 109
ppb = mg / kL = 0.001 g / 1,000,000 mL
Final report is to include answers to the following questions:
Introduction to the Scientific
Literature Part II Answer the following questions from reading the JACS publication [J. Am. Chem. Soc., 123, 1625 (2001)] (pdf) Part II) Coordination Chemistry 1. a) Write a balanced equation for the reaction of HAuCl4 plus AgNO3 plus CH3CH2SCH2CH2Cl.
|
Stereochemistry & Coordination Complexes
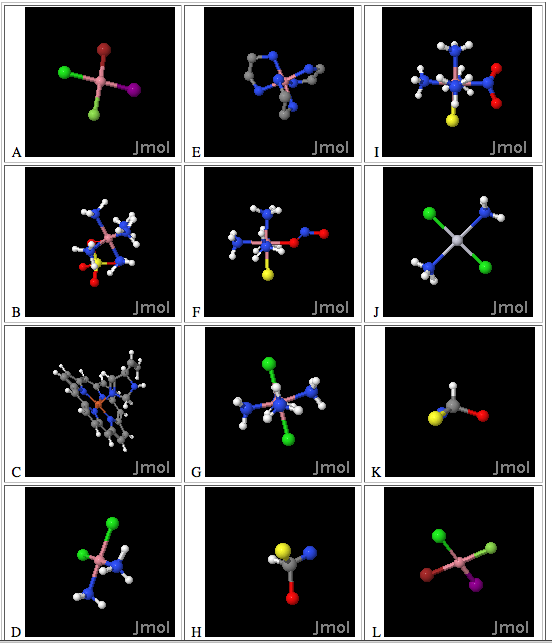
Click above to link to the jmol files: http://chemconnections.org/jmol-pdb/coord-ex1.html, view the molecules in 3-D, and match their respective letters to the descriptions below. (Turn in Form .pdf)
1. Identical molecules: _______ and _________
2. Enantiomers: _______ and _________
3. Linkage isomers: _______ and _________
4. An optically active octahedral complex: _______
5. Pentaamminesulfatocobalt(III) _______
6. Mirror images: _______ and _________
7. A metallo porphyrin: _______
8. A cis square planar complex: _______
9. A cis octahedral complex: _______
10. A trans square planar complex: _______
11. A trans octahedral complex: _______
12. A tetrahedral molecule: _______
13. [Co(NH3)4Cl2]+ : _______
14. Coordination number = 5: _______
15. Coordination number = 4, sp3d2 hybridization, low spin: _______
Kinetics: Rate Law Determination; Crystal Violet, a.k.a. “Gentian”
Background / Instructions .pdf
Perkin set up a factory on a 6-acre site in West London. At the Royal Exhibition of 1862, Queen Victoria made an appearance in a silk gown, which was dyed with mauveine that was made in the late 1850s. She unveiled a "penny lilac" postage stamp thought to have been dyed with the same compound. Curiously, the "correct" structure for mauveine was only determined in 1994, and a complete stereochemical synthesis of quinine was completed by Gilbert Stork in 2001.
The color mauve fell from fashion in the late 1860s, but Perkin discovered other new dyes. It was said that the water in a canal near his factory turned a different color every week, depending on what dyes were being developed at the time.
In the textile industry, almost 100 liters of water are used to dye a kilogram of fiber. Today, the aqueous effluent from the dyeing processes must be treated, but most local regulations in the United States allow the water to be returned to its natural source (often a local stream or river) without complete removal of all of the chemicals used in the dyeing process.
Many fibers, such as cotton, nylon, and polyesters, absorb large amounts of water. The dyeing process is very energy intensive because the water that is evaporated has an extremely high heat of vaporization . Besides coloring textiles, dyes have been used as acid-base indicators, histological stains for cells, and as fluorophores in immunoassays.Crystal violet (gentian) is within this class of molecules. It reacts relatively slowly under basic conditions loosing its deep purple color to form colorless solutions. This experiment will determine the rate law and rate constant for the reaction under basic conditions with excess hydroxide ion as the reactant.
Refer to the DVC lab manual pg. 61 and the procedure on pg. 62. Part 3 will not been done. The Beer’s Law for part 3 follows.
Figure 1 below shows a beam of light (electromagnetic radiation) before and after it has passed through a solution having a path length, b, and a concentration, c. The power of the beam is attenuated (diminished) from Po to P because of the interactions of the photons and the absorbing particles. Transmittance of the solution is the fraction of light that passes through the solution, P / Po. In infrared spectroscopy it is often plotted as percent transmission versus frequency (cm-1).
Absorbance ( A ) is expressed as a logarithmic function where the absorbance of a solution increases exponentially as attenuation of the beam becomes larger (less transmittance). Absorbance is directly proportional to the path length, b, through the solution and the concentration, c, of the absorbing species shown in the equation: A = abc, where a is a proportionality constant called absorptivity. If the concentration is expressed in molarity (M) and the cell length in centimeters, the absorptivity is called the molar absorptivity and given the symbol ε,. It has also been referred to as the molar extinction coefficient.A = εbc is known as Beer’s Law.
Beer’s Law will be used to quantitatively determine the respective concentrations of crystal violet from measured absorbances at different times during the reaction of crystal violet with hydroxide ion. This data will be plotted against time and processed as instructed on pp. 64-65. Calculations are to include items #10, 11, and 15-19.
Results/ Conclusions are to include: the integrated rate law for each run using the pseudo rate constant, the overall rate law and rate constant, the order of each reactant, the overall order of reaction, and the 1⁄2 life for the reaction.
KINETICS: Energy of Activation
BrO3 + Br- + 6 H+ -> Br2 + 3 H2O
The following data is a compilation of several students' experimental results for the disappearance of red color in the phenol/methyl red monitored bromide/bromate reaction using a different range of temperatures than those in the lab manual's experimental procedure.
Part I:
- Using Excel plot this data as ln t vs 1/T(K), clearly label the plot, and determine Ea for the reaction.
- Write the half reactions for the oxidation and reduction reactions in the overall reaction, provide the reduction potentials of each half reaction, and clearly identify which is oxidation and which is reduction.
- How many electrons are transferred in the process?
- Is the reaction spontaneous? Show a calculation for the standard potential (Voltage) of the reaction.
| Kinetics Data: | |||||
| 0 oC | 10 oC | 20 oC | 30 oC | 40 oC | |
sec
|
408 | 335.4 | 135.5 | 61.2 | 33.9 |
sec
|
220 | 321.6 | 161.6 | 69.6 | 42.65 |
sec
|
1997 | 130.8 | 84.0 | 39.1 | 39.2 |
sec
|
578.2 | 345.5 | 112 | 158.2 | 88.8 |
sec
|
691 | 295 | 163.8 | 116.9 | 39.0 |
sec
|
498 | 232 | 1997 | 59.3 |
36.0
|
sec
|
543 | 270 | 123.4 | 84.6 | 41.6 |
sec
|
623 | 371 | 175.5 | 135 | 35.8 |
sec
|
444 | 323 | 126.4 | 76.5 | 34.3 |
sec
|
171.5 | ||||
sec
|
268 | ||||
sec
|
167
|
Introduction to the Scientific Literature
Reading and Understanding a PublicationAnswer the questions in the handout from reading the JACS paper [J. Am. Chem. Soc., 123, 1625 (2001)] (.pdf) and the Web article: Soluble Gold Catalyst
Part II:
1) Write the proposed rate law by the authors.
2) What is the overall order of reaction proposed by the authors?
3) What is the value for the observed rate constant (including units)?
4) Write the reaction for the rate limiting step in the mechanism.
Turn in form .pdf
Calibrated Peer Review (CPR) Ozone Writing Assignment: http://cpr.molsci.ucla.edu/
BEWARE of Plagiarism1) Take the Tour if you have not already done so.
2) For first time login: Select Diablo Valley College from Institutions, login using your DVC id for initial login, record your new CPR id and change your password.
3) Begin assignment following the scheduled due dates.
IR / NMR / Structural Elucidation:
http://www2.chemistry.msu.edu/faculty/reusch/VirtTxtJml/Spectrpy/InfraRed/infrared.htm#ir1
http://www.chem.ucla.edu/~webspectra/http://www.chemaxon.com/marvin/sketch/index.php

Aspirin: Synthesis/ Spectroscopic Analysis/ Structural Elucidation/ Purity
Synthesis: Prelab A; Prelab B
IR Prelab UnknownsSpectroscopic Analysis/Structural Elucidation
IR / NMR
Purity of aspirin; Beer's Law Plot / Colorimetry
a) Weigh to 4 decimal places approximately 0.4g of reagent grade acetylsalicyclic acid, add 10ml of 1M sodium hydroxide and carefullyheat the solution to boiling. Caution: Sodium hydroxide is highly caustic, wear eye protection and handle carefully. Avoid spattering material on boiling. Allow to cool, completely transfer all of the material to a 250ml volumetric flask and dilute to the mark with distilled water.
b) Pipet 5ml to a l00ml volumetric flask and dilute with 0.02M FeCl3 solution, Iron (III) chloride which is buffered to pH 1.6 with HCL/KCl. Prepare 4 other solutions as above by pipeting lml, 2ml, 3ml and 4ml aliquots respectively of the 250ml sodium acetyl salicylate stock solution and diluting to l00ml with the buffered Fe(III) solution.c) Using the Fe(III) solution to calibrate the spectrophotometer, determine the absorbance (A) of each dilution at 530 nm and calculate the Molarity (M) of each. Plot (A) vs (M) and draw the best possible straight line.Quantitative determination of the purity of the synthetic sampleand an unknown commercial aspirin sample.
d) Follow the procedure as in IIIa) using your synthetic aspirin sample; separately repeat this same procedure for an unknown sample of a commercial product which Dr. R. will provide. The commercial sample may appear a bit cloudy due to insolble binders in the tablet, but this will not affect your results.
Make a single dilution of 1ml for each of the 2 stock solutions as in III.b) using a 25mL volumetric flask to prepare each of the FeCl3 solutions. Determine the absorbance of each. Find the absorbance value on the Beer's Law plot prepared from the data in III.c) and determine the corresponding concentration (M). Using the molarity calculate the amount (mg) of aspirin present in the commercial sample; also calculate the % purity of your synthetic sample, that is, the amount aspirin present / amount weighed to make up the original stock solution x 100.
BEER'S LAW DATA Mass (Weight) Container + Aspirin 13.2217 Container 12.8181 1.00 0.0935 2.00 0.1669 3.00 0.2541 4.00 0.332 5.00 0.4089
Aspirin Analysis Commer. Unk. Commer. Unk. Synthetic Synthetic Bay-330 SKF-202 DIY-121a DIY-121b mass (grams) 0.3999 0.3555 0.3812 0.3658 absorbance 0.07 0.171 0.064 0.234
WebMO: http://butane.cabrillo.edu/
WebMO Tutorial pdf
WebMO Worksheet pdf
VSEPR (Images and Valence Shell Electron Pair Repulsion "Theory")
http://chemconnections.org/VSEPR-jmol/
http://chemconnections.org/general/chem120/VSEPR/
Molecular Orbitals .pdf
Calibrated Peer Review (CPR): VSEPR Writing Assignment
http://cpr.molsci.ucla.edu/ BEWARE of Plagiarism1) Take the Tour if you have not already done so.
2) For first time login: Select Diablo Valley College from Institutions, login using your DVC id for initial login, record your new CPR id and change your password.
3) Begin assignment following the scheduled due dates.
Proliferation of Nuclear Weapons (pdf)
The “New Start Treaty”, which was ratified by the US Senate in December 2010, has served to further reduce the US nuclear arsenal. The largest US nuclear weapon, B53, which had the destructive power equivalent to the explosion of 9 megatons of TNT (9 x 109 kg; 600 times larger than the Hiroshima bomb) and containing about 125 kg of conventional and fissionable explosives, was decommissioned in October 2011.
Your team has been assigned by the Director of Homeland Security to investigate a scenario that a rogue nation, Kabaamistan, with a militant group of educated scientists, “De.M” (Derriéres Mal), might be developing a nuclear device that could be detontated within the U.S. or Europe, key targets in their strategic global plans.
Your team is to provide clear, concise answers to the following questions, and an appraisal of the possibility and probability of such a threat. Refer to the links and information found at: http://chemcases.com/nuclear/nccases.htm and http://topics.nytimes.com/top/news/science/topics/atomic_weapons/index.html
and the article:
http://chemconnections.org/general/chem121/Nuclear/Nu-weap-Econ-07.pdf
Your team is to turn-in written answers to questions #1, #2, #3, and #4, and present your overall appraisal including answers to questions #5-#8 to the class.
1. What are the necessary components of such a device?
2. Would development of a Pu-239 or U-235 device be the most likely?
3. How much fissionable material would be required?
4. How would this material be produced or acquired? Which is more likely, production or acquisition?
5. Would transporting / delivering the device to the US or Europe be feasible?
6. What is the likelihood of this happening?
7. Are there other nuclear weapon scenarios that should be considered as serious threats?
8. What measures can be taken to reduce the nuclear risks?
Your group will have 10 minutes to orally report your appraisal, and then be prepared to answer questions after the presentation.
You must be present for the presentations to receive credit for the assignment. You will confidentially rate your own performance, the performance of the other members of your group, and the oral reports of the other groups.
Oxidation-Reduction / Titration Lab:
Ethanol Analysis:Alcoholic Beverage Analysis;Ethanol Article; Applied Questions
End of Course Survey:
(Anonymous survey to be completed prior to final exam])
http://www.wcer.wisc.edu/salgains/student/Course ID:
Course Password: chem121
Login: Enter your DVC ID # as ID for course